Answer:
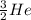
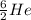
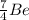
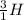
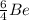
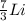
Step-by-step explanation:
In the first nucleus we are told that there are two protons and one neutron. Let us remember that the mass number = number of protons + number of neutrons.
This implies that, for the first specie the mass number is 3, for the second specie the mass number is 6 and the third specie has a mass number of 7 and so on. The mass number is indicated as a superscript.
The atomic number is the number of protons in the nucleus of the atom and helps us to identify the atom. It is always written as a subscript as shown.