Answer:
1750mL
Explanations:
In order to get the required volume, we will use the dilution formula expressed as:
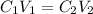
where:
C₁ and C₂ are the initial and final concentration respectively
V₁ and V₂ are the initial and final volume respectively
Given the following parameters:
C₁ = 500mg/L
C₂ = 100mg/L
V₁= 350mL
Required
V₂
Substitute the given parameters into the formula to have:
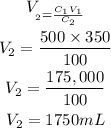
Hence the amount of water needed is 1750mL