Answer:
507 m/s
Step-by-step explanation:
To determine the speed of oxygen molecules in the air at a temperature of 56.7 °C (134 °F), we use the root mean square speed formula for gas molecules.. The formula for the average speed of gas molecules is given by:
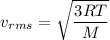
Where,
- 'v' is the average speed of the molecules
- 'R' is the gas constant (≈ 8.31 J/mol·K)
- 'T' is the absolute temperature in Kelvin,
- 'M' is the molecular mass of the gas.
We are given,
- T = 56.7 °C (56.7 + 273.15 = 329.85 K)
- M = 2(15.999 g/mol) = 31.998 g/mol O₂ (this will need to be converted to kg/mol)
Plug our given values into the formula:
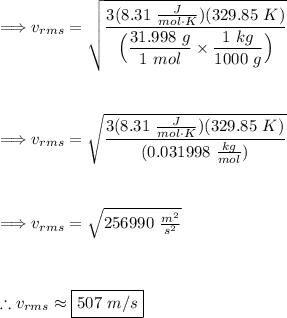
Thus, we get a speed of approximately 507 m/s.