Answer:
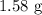
Step-by-step explanation:
10.11 g sample of
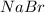 contains 22.34%
contains 22.34%
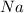 by mass
by mass
According to the law of constant composition the if one sample of
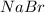 has 22.34% of
has 22.34% of
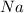 by mass then any other sample of
by mass then any other sample of
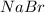 will have the same percentage of the amount of
will have the same percentage of the amount of
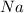 .
.
For a sample of 7.09 g we have
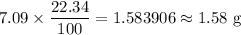
The mass of sodium in the required sample is
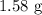 .
.