To balance an equation with the oxidation numbered method we must write the oxidation states of the elements that participate in the reaction.
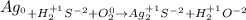
Once we have the oxidation states we must identify how they change from reactants to products. Thus we identify which are oxidized and which are reduced. If its oxidation state increases it is because the element is oxidized, if it decreases it will have been reduced.
We see that the elements that change their oxidation state are silver, Ag and Oxygen. We have that: