Answer:
21.614 grams are found in
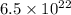 molecules of caffeine.
molecules of caffeine.
Step-by-step explanation:
The chemical formula for caffeine is
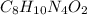 , the molar weight of caffeine is:
, the molar weight of caffeine is:
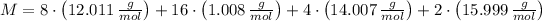
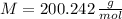
According to Avogadro's Law, a mol of caffeine contains
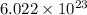 molecules. The number of grams contained in
molecules. The number of grams contained in
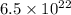 molecules is determined by simple rule of three:
molecules is determined by simple rule of three:
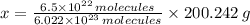
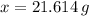
21.614 grams are found in
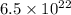 molecules of caffeine.
molecules of caffeine.