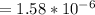Answer:
Kb
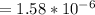
Step-by-step explanation:
The correct question is
What is Kb for N2H4 if the pH of a 0.158M solution of N2H5Cl is 4.5?
Solution-
N2H4Cl hydrolyses on addition of water
The reaction equation is as follows -
N2H4+ + H2O ----> N2H4 + H3O+
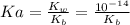
pH
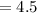
![[H+] = 10^(-pH) = 10^(-4.5)](https://img.qammunity.org/2022/formulas/chemistry/college/vk315p6as11olrl9nty197ttrlqj6i9hmn.png)
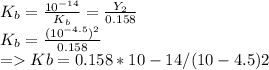
Kb