Answer:
16.09 kg
Step-by-step explanation:
To solve this problem first we convert those 5.0 gallons of ethanol into liters:
- 5.0 gallons *
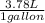 = 18.9 L
= 18.9 L
Now we calculate the mass of that volume of ethanol, using its density:
We convert 18.9 L ⇒ 18.9 * 1000 = 18900 mL
- Mass = 0.7893 g/mL * 18900 mL = 14917.77 g
Finally we convert the masses of the bottle and shipping container and packaging and add them to the mass of ethanol:
- 1.82 lb * 453.592 = 825.54 g
- 0.76 lb * 453.592 = 344.73 g
- Total Mass = 14917.77 g + 825.54 g + 344.73 g = 16088.04 g
So the shipping weight would be 16088.04 g, or 16.09 kg