ANSWER
Step-by-step explanation
Given information
The mass of calcium bromide is 5.0 grams
Follow the steps below to find the mole of calcium bromide
Step1: Write the formula for calculating mole
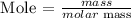
Recall, that the molar mass of calcium bromide is 199.89 g/mol
Step 2: Substitute the given data into the formula in step 1
![undefined]()