The concentration of magnesium in the solution is approximately 0.550 milliequivalents per milliliter.
To express the concentration of magnesium in the magnesium citrate laxative solution (CITROMA) as milliequivalents per milliliter, we need to follow these steps:
1. Understand the Composition: We know that the solution contains 1.745 grams of magnesium citrate per fluid ounce.
2. Determine the Magnesium Content in Magnesium Citrate: Magnesium citrate is a compound containing magnesium, carbon, and oxygen. The molecular formula for magnesium citrate is
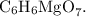 The molar mass of magnesium citrate can be calculated by adding the molar masses of all atoms in the formula. The molar mass of magnesium (Mg) is approximately 24.305 g/mol.
The molar mass of magnesium citrate can be calculated by adding the molar masses of all atoms in the formula. The molar mass of magnesium (Mg) is approximately 24.305 g/mol.
3. Calculate the Molar Mass of Magnesium Citrate: The molar mass of magnesium citrate can be calculated as follows:
- Carbon (C): 12.01 g/mol × 6 atoms = 72.06 g/mol
- Hydrogen (H): 1.008 g/mol × 6 atoms = 6.048 g/mol
- Magnesium (Mg): 24.305 g/mol × 1 atom = 24.305 g/mol
- Oxygen (O): 16.00 g/mol × 7 atoms = 112.00 g/mol
- Total = 72.06 + 6.048 + 24.305 + 112.00 = 214.413 g/mol
4. Find the Proportion of Magnesium in Magnesium Citrate: This is the ratio of the molar mass of magnesium to the molar mass of magnesium citrate.
5. Convert Fluid Ounces to Milliliters: There are approximately 29.5735 milliliters in one fluid ounce.
6. Calculate the Mass of Magnesium per Milliliter: Multiply the mass of magnesium citrate per fluid ounce by the proportion of magnesium, and then convert to milliliters.
7. Convert Mass to Moles: Since 1 mole of magnesium has a mass of 24.305 grams, convert the mass of magnesium per milliliter to moles.
8. Convert Moles to Milliequivalents: One equivalent of a substance is its molar mass divided by its valence. Magnesium has a valence of +2. Therefore, 1 milliequivalent is
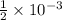 moles of magnesium.
moles of magnesium.
9. Final Calculation: Calculate the number of milliequivalents of magnesium per milliliter of solution.
Now, let's perform these calculations.
The concentration of magnesium in the magnesium citrate laxative solution (CITROMA) can be expressed in milliequivalents per milliliter as follows:
1. The molar mass of magnesium citrate
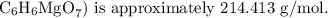
2. The proportion of magnesium in magnesium citrate is calculated using the molar mass of magnesium and magnesium citrate. The calculated mass of magnesium per milliliter of solution is approximately 0.00669 grams.
3. Finally, converting this mass to milliequivalents, we find that the concentration of magnesium in the solution is approximately 0.550 milliequivalents per milliliter.