Question 16:
We are given the following balanced equation: (Remember to always balance the equation)
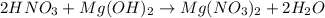
We are also told that Mg(OH)2 is in excess, meaning HNO3 is the limiting reactant, we will use its moles to find the number of moles of Mg(NO3)2, then we can convert that to mass.
The ratio between HNO3 and Mg(NO3)2 is 2:1
Therefore the number of moles of Mg(NO3)2 = 8.00 mol x (1/2) = 4.00 mol
Now we can convert the number of moles of Mg(NO3)2 to mass: molar mass of Mg(NO3)2 = 148,3 g/mol
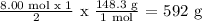
So the mol will cancel the mol, then you will be left with g.