Final Answer:
(a) The final pressure of the gas is
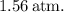
(b) The initial temperature is
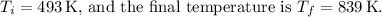
(c) The heat transfer during this process
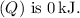
(d) The change in internal energy
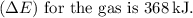
(e) The work done by the gas
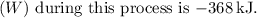
Step-by-step explanation:
In an adiabatic process, there is no heat exchange with the surroundings
 The relationship between initial and final pressure
The relationship between initial and final pressure
 volume
volume
 for an adiabatic process involving an ideal gas is given by the equation:
for an adiabatic process involving an ideal gas is given by the equation:
![\[ P_i V_i^\gamma = P_f V_f^\gamma \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/em0tphl9tv01krugv1sxhn2yokiaf0c5ih.png)
where
 is the heat capacity ratio (ratio of specific heat at constant pressure to specific heat at constant volume). For a diatomic ideal gas,
is the heat capacity ratio (ratio of specific heat at constant pressure to specific heat at constant volume). For a diatomic ideal gas,
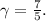
(a) Solving for
 , we find the final pressure to be
, we find the final pressure to be
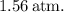
(b) To find the initial and final temperatures, we can use the ideal gas law
 is the number of moles. Rearranging for temperature,
is the number of moles. Rearranging for temperature,
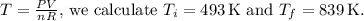
(c) As the process is adiabatic
 there is no heat transfer.
there is no heat transfer.
(d) The change in internal energy
 can be calculated using the first law of thermodynamics:
can be calculated using the first law of thermodynamics:
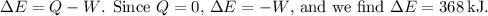
(e) The work done (\(W\)) is the area under the pressure-volume
 The negative sign indicates work done by the gas on its surroundings.
The negative sign indicates work done by the gas on its surroundings.