ANSWER
ΔE = 70 J
Step-by-step explanation
Given:
• The work done on the system, W = 48 J
,
• The heat absorbed by the system, Q = 22 J
Find:
• The change of energy of the system, ΔE
We will use the convention where:
• Heat transferred to the system: positive (otherwise negative)
,
• Work done on the system: positive (otherwise negative)
By the first law of thermodynamics,
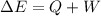
Replace with the known values and solve,
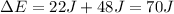
Hence, the change in the system's internal energy is 70 J.