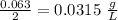Answer:
Follows are the solution to the given points:
Step-by-step explanation:
In part 1:
As described and in the query, they become precipitated whenever the solutions are refrozen to
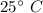 .
.
Afterward, certain precipitate becomes replaced as well as the remaining water is evaporated, it implies that certain precipitate remained throughout the solution to just the container when the entire balance is evaporated.
The unrecoverable salt precipitates whenever the solvent is cooled at
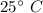 and the remaining salt dissolves. It dissolved salt remains whenever the water is evaporated because as dissolved salt value is given that results can be achieved.
and the remaining salt dissolves. It dissolved salt remains whenever the water is evaporated because as dissolved salt value is given that results can be achieved.
In part 2:
They have precipitation weight =
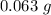 . They have a
. They have a
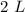 the solution, they may disregard the volume increases due to its precipitation. The intensity therefore is
the solution, they may disregard the volume increases due to its precipitation. The intensity therefore is