Answer:
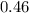 moles of water were removed from the sample by the heating process
moles of water were removed from the sample by the heating process
Step-by-step explanation:
Given
Mass of the evaporating dish
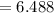 grams
grams
Mass of the sample of hydrate
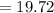 grams
grams
Mass of the sample and dish after heating
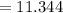 grams
grams
The weight of water removed through evaporation
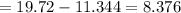 grams
grams
Weight of one mole of water
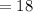 grams
grams
Number of moles of water in
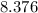 Grams of water is equal to
Grams of water is equal to
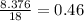 moles
moles