Answer
1.775 M
Step-by-step explanation
Given:
Volume of solution, V = 400.0 mL
Mass of NaCl = 45.5 g
What to find:
The molarity of the solution.
Step-by-step solution:
Step 1: Convert the volume from mL to L.
This can be done by dividing the volume in mL by 1000.
V = 400.0 mL = (400.0/1000) = 0.4L
Step 2: Convert the mass of NaCl to moles.
The mass can be converted to moles using the mole formula below:
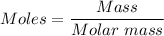
From the periodic table, the molar mass is determined to be = 58.44 g/mol
Putting mass = 41.5 g and molar mass = 58.44 g/mol into the formula, we have:
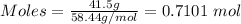
Step 3: Calculate the molarity of the solution using the molarity formula.
Molarity formula is given by:
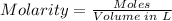
Moles= 0.7101 mol and V = 0.4 L
Therefore,
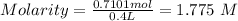
The molarity of a 400.0 mL solution that contains 41.5 g of NaCl = 1.775 M.