Final answer:
The maximum temperature in a cold air-standard Otto cycle can be determined using the given information and the equation
 constant. The maximum temperature is found to be 1711.55°R.
constant. The maximum temperature is found to be 1711.55°R.
Step-by-step explanation:
The maximum temperature in a cold air-standard Otto cycle can be determined using the given information. The compression ratio is 6, and the temperature at the beginning of the compression process is 520°R. In an Otto cycle, the maximum temperature occurs at the end of the compression process when the gas is at its smallest volume. The relationship between temperature and volume for an adiabatic process is described by the equation
 constant, where T is the temperature, V is the volume, and y is the specific heat ratio. Since the specific heat ratio for air is 7/5, we can use this equation to find the maximum temperature.
constant, where T is the temperature, V is the volume, and y is the specific heat ratio. Since the specific heat ratio for air is 7/5, we can use this equation to find the maximum temperature.
Using the given values, we can set up the equation as follows:
Since the compression ratio is 6, V1/V2 = 6, so we can substitute 6 for V1/V2:
(520°R)
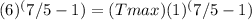
Simplifying the equation, we get:
520°R *
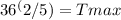
Calculating the right side of the equation, we find:
520°R * 3.3019 = Tmax
Tmax = 1711.55°R
Therefore, the maximum temperature in the cold air-standard Otto cycle is 1711.55°R.