We are asked to find the pressure of a gas that is in certain conditions. To find it we must assume that there is no interaction between the gas molecules and the gas behaves like an ideal gas, in this way we can apply the ideal gas law that tells us:
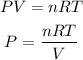
Where,
P is the pressure of the gas
V is the volume of the gas = 15L
T is the temperature of the gas = 400K
n is the number of moles = 5mol
R is a constant = 0.08206 (atm L)//(mol K)
Now, we replace the known data in the equation:
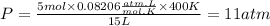
Answer: The pressure of the gas will be 11 atm