Answer:
Lithium oxide, Li₂O.
Step-by-step explanation:
Hello!
In this case, according to the given amounts, it is possible to write down the chemical reaction as shown below:
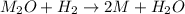
Which means that the metallic oxide has the following formula: M₂O. Next, we can set up the following proportional factors according to the chemical reaction:
Thus, we perform the operations in order to obtain:
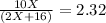
So we solve for x as shown below:
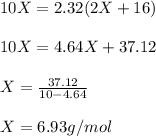
Whose molar mass corresponds to lithium, and therefore, the metallic oxide is lithium oxide, Li₂O.
Best regards!