Answer:
V = 502 L
Step-by-step explanation:
Gas Stoichiometry
We're given:
CH₄(g) + 2O₂(g) → CO₂(g) + 2 H₂O(l)
First, determine the moles of CO₂ using this ratio:

Now, to determine its volume, use the ideal gas law:

⇒ Isolate V
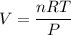
⇒ Convert given temperature to Kelvin


⇒ Round to significant figures

Therefore, the volume of CO₂ produced is 502 L.