Answer:
0.0220 g (22 mg) of NO2.
Step-by-step explanation:
To solve this type of problem, it is best to work with grams. Remember that 1 kg equals 1000 g and 1 g equals 1000 mg. The conversion for 5.26 kg of nitrogen monoxide (NO) would be:
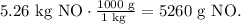
And for 7.64 mg of oxygen (O2) is:
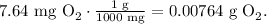
The next step is to find the number of moles of each reactant using its molar mass. The molar mass of NO is 30 g/mol (you can calculate the molar mass of a compound using the periodic table):
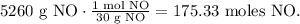
And the moles of oxygen (O2) is 32 g/mol:
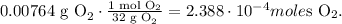
The next step is to see how many moles of NO" can be produced for each reactant.
You can see in the chemical equation that 2 moles of NO produce 2 moles of NO2, so the molar ratio between them is 1:1. This means that 175.33 moles of NO reacted produce 175.33 moles of NO2.
Now, you can see that 1 mol of O2 reacted produces 2 moles of NO2, so let's see how many moles of NO2 are being produced:
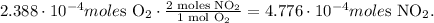
You can note that the limiting reactant, in this case, is oxygen (O2) because this reactant imposes the "limit" to produce the product.
The final step is to convert from 4.776 x 10^(-4) moles of NO2 to grams using its molar mass which is 46 g/mol. The conversion will look like this:
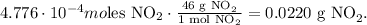
We obtain 0.0220 g (22 mg) of NO2 from 5.26 kg of NO and 7.64 mg of O2.