Answer:
0.392 moles of KNO3.
Step-by-step explanation:
To find the moles of a solute based on the volume and concentration of a solution, we use the following formula:
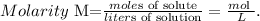
The given data is: molarity = 0.560 M and volume (liters of solution) = 0.70 L. So, let's solve for 'moles of solute' and replace the values that we have. The solute in this case, would be KNO3:
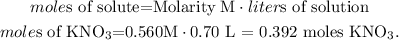
We're going to use 0.392 moles of KNO3 to prepare 0.70 L of a 0.560 M solution.