Answer: The molar mass of menthol is 156.15 g/mol
Step-by-step explanation:
Depression in freezing point is given by:
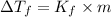
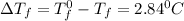 = Depression in freezing point
= Depression in freezing point
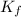 = freezing point constant =
= freezing point constant =
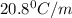
m= molality
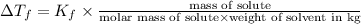
Weight of solvent (cyclohexane)= 25.0 g = 0.025 kg
Molar mass of solute (menthol) = ?
Mass of solute (menthol) = 0.533 g
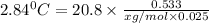
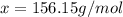
The molar mass of menthol is 156.15 g/mol