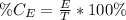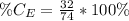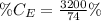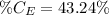Answer:
See Explanation
Step-by-step explanation:
The question is incomplete; as the mixtures are not given.
However, I'll give a general explanation on how to go about it and I'll also give an example.
The percentage of a component in a mixture is calculated as:
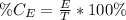
Where
E = Amount of element/component
T = Amount of all elements/components
Take for instance:
In
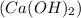
The amount of all elements is: (i.e formula mass of
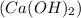 )
)
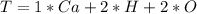
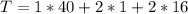
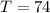
The amount of calcium is: (i.e formula mass of calcium)
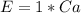
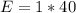
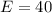
So, the percentage component of calcium is:
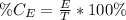
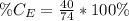
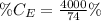
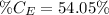
The amount of hydrogen is:
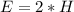
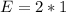
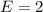
So, the percentage component of hydrogen is:
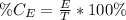
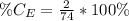
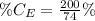
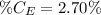
Similarly, for oxygen:
The amount of oxygen is:
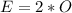
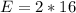
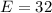
So, the percentage component of oxygen is: