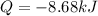0.0We are asked to determine the volume of a gas given the moles, the temperature, and the pressure. To do that, we use the following formula:

Where:

Now, we substitute the values for the final state. Since the process is isothermally this means that the final temperature is the same as the initial temperature. we get:
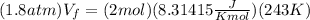
We need to convert the pressure from atmospheres to Pascals. To do that we use the following conversion factor:
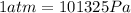
Multiplying by the conversion factor we get:
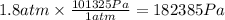
Now, we substitute the value in the formula:
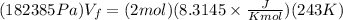
Solving the operations:
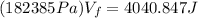
Now, we divide both sides by 182385Pa:
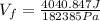
Solving the operations:
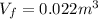
Therefore, the final volume is 0.022 cubic meters.
Part B. We are asked to determine the work done. To do that we will use the formula for isothermic work:
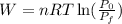
Where:
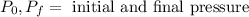
Now, we plug in the values:
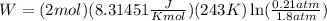
Now, we solve the operations:
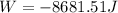
Therefore, the work done is -8681.51 Joule. To convert to kilojoules we divide by 1000:
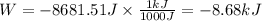
Part 3. In an isothermic process the change in internal energy is zero, therefore, according to the first law of thermodynamics we have:
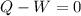
Therefore:
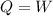
Therefore, the amount of heat is equal to the amount of work. Therefore, the thermal energy transferred is: