The molar concentration, often called molarity, describes how much of a substance (a solute) is present per unit of solvent. By definition, the molar concentration (M) is equal to the number of moles (n) of solute divided by the number of liters (the volume, or V) of the solution.
Here, your solute is potassium nitrate, or KNO3. You're given the mass of KNO3 (505 g), but you need to convert this quantity to moles before you can find the molarity. To go from mass to moles, simply divide the mass of the substance by its molar mass (given to you as 101.1 g/mol).
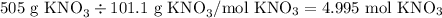
Now that we have the moles of solute, we divide by the liters of solution. We're given the volume of solution in milliliters, so to convert to liters, simply divide by 1000 (1 L = 1000 mL, so 1 mL = 1/1000 mL). Our volume of solution is thus 0.250 L.
Finally, we can calculate the molar concentration of the KNO3 solution:
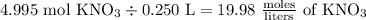
But, we're told to round our answer to three sig figs. Thus, our rounded and final answer would be 20.0 moles/liters of KNO3.