This is a two-step question. First, convert the mass of NO2 into the number of moles of NO2. Secondly, quantify the number of moles of NO2 in terms of the number of moles of NO2. The steps and answer are as follows
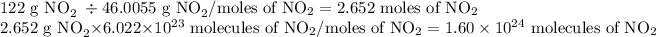
The answer has been provided to three significant figures to correspond with the number of sig figs given in the mass.