Answer:
a) 0.33 M
b) 2.63x10^-14 M
Step-by-step explanation:
1st) It is necessary to write and balance the chemical reaction:

From the balanced reaction we can see that 1 mole of HClO4 reacts with 1 mole of NaOH.
2nd) Since the relation between the acid (HClO4) and the base (NaOH) is 1:1, the 0.10 moles of NaOH will react with 0.10 moles of acid:
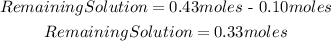
So, in the solution 0.33 moles of HClO4 will remain unreacted, and the concentration of H3O+ will be 0.33M.
3rd) In this case we can proceed the same as in the first part:
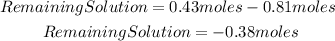
In this case, the addition of 0.81 moles of NaOH neutralizes all the acid in the solution and 0.38 moles of the base will remain unreacted.
So, the concentration of OH- will be 0.38M, and it is necessary to calculate the pOH of the solution, because when OH- is left over, the solution will be basic:
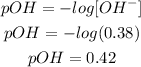
The pOH of the solution is 0.42.
4th) Now with the pOH we can calculate the pH of the solution, with the following formula:
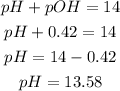
The pH of the solution is 13.58.
5th) Finally, with the value of pH and the pH formula, we can calculate [H3O+]:
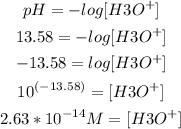
So, the concentration of H3O+ is 2.63x10^-14M.