Answer:
4. 2.4
5.
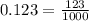
6. IV
Explanation:
4. 3 < 5, so we don't add a tenth when rounding. Hence the answer is 2.4
5.
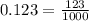 . Because
. Because
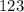 isn't divisible by 2 or 5, we know that this is the most simplified form.
isn't divisible by 2 or 5, we know that this is the most simplified form.
6. Putting the I before the V negates the I. So IV = 5 - 1 = 4.