Answer:
d) The oxidation number of K in K2SO3 is +1.
e) The oxidation number of S in K2SO3 is +4.
f) The oxidation number of O in K2SO3 is -2.
g) The oxidation number of S in S8 is 0.
Step-by-step explanation:
In each case, we can use the Periodic Table of Elements to consult the oxidation number of the elements.
d) In this case, K is in the group 1 of the Periodic Table, so it has always an oxidation number of +1.
f) Oxygen (O) always has an oxidation number of -2 (except in peroxides which has -1).
e) Knowing that the molecule of K2SO3 is neutral (the total charge of the molecule is equal 0), we can sum all the charges of the elements in the molecule, to find out which oxidation number has S in this molecule:
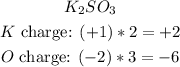
Now we have to sum them and find out the oxidation number of S that will made the total charge of the molecule equal 0:
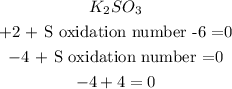
So, the oxidation number of S is +4.
g) Finally, in this case, the oxidation number of S in S8 is 0, because it is a pure compound.