Answer:

Step-by-step explanation:
The number of moles of
 can be found by using the formula;
can be found by using the formula;
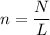
where n is the number of moles
N is the number of entities
L is the Avogadro's constant which is
6.02 × 10²³ entities
From the question;
N = 6.91 × 10³⁶ formula units
