Answer:
The energy required is 158 J
Step-by-step explanation:
Calorimetry is the measurement and calculation of the amounts of heat exchanged by a body or a system.
When the heat added or removed from a substance causes a change in temperature in it without affecting its molecular structure, this heat is called sensible heat.
In other words, when a system absorbs (or gives up) a certain amount of heat, it can happen that it experiences a change in its temperature, which implies sensible heat.
The equation that allows calculating heat exchanges is:
Q = c * m * ΔT
where Q is the heat exchanged by a body of mass m, made up of a specific heat substance c and where ΔT is the temperature variation.
In this case:
- c=0.79
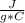
- ΔT= Tfinal - Tinitial= 50 C - 30 C= 20 C
Replacing:
Q= 0.79
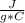 *10 g* 20 C
*10 g* 20 C
Solving:
Q= 158 J
The energy required is 158 J