Answer:
8740 joules are required to convert 20 grams of ice to liquid water.
Step-by-step explanation:
The amount of heat required (
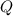 ), measured in joules, to convert ice at -50.0 ºC to liquid water at 0.0 ºC is the sum of sensible heat associated with ice and latent heat of fussion. That is:
), measured in joules, to convert ice at -50.0 ºC to liquid water at 0.0 ºC is the sum of sensible heat associated with ice and latent heat of fussion. That is:
![Q = m\cdot [c\cdot (T_(f)-T_(o))+L_(f)]](https://img.qammunity.org/2022/formulas/chemistry/high-school/ey8jjdk6iquhajfp9unfcgnd2hwru6gd99.png) (1)
(1)
Where:
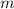 - Mass, measured in grams.
- Mass, measured in grams.
 - Specific heat of ice, measured in joules per gram-degree Celsius.
- Specific heat of ice, measured in joules per gram-degree Celsius.
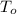 ,
,
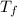 - Temperature, measured in degrees Celsius.
- Temperature, measured in degrees Celsius.
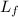 - Latent heat of fussion, measured in joules per gram.
- Latent heat of fussion, measured in joules per gram.
If we know that
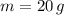 ,
,
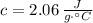 ,
,
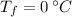 ,
,
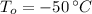 and
and
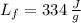 , then the amount of heat is:
, then the amount of heat is:
![Q = (20\,g)\cdot \left\{\left(2.06\,(J)/(g\cdot ^(\circ)C) \right)\cdot [0\,^(\circ)C-(-50\,^(\circ)C)]+334\,(J)/(g) \right\}](https://img.qammunity.org/2022/formulas/chemistry/high-school/40rbqywioom7wyqqhrg2t0lcfjbza2e3i6.png)
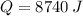
8740 joules are required to convert 20 grams of ice to liquid water.