V₂=0.894 L
Further explanation
Given
V₁=1.55 L
T₁=27 + 273 = 300 K
P₁=1 atm
T₂=-100+273 = 173 K
Required
The final volume(V₂)
Solution
Charles's Law
When the gas pressure is kept constant, the gas volume is proportional to the temperature
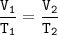
Input the value :
V₂=V₁T₂/T₁
V₂=1.55 x 173/300
V₂=0.894 L