The given question is incomplete. The complete question is:
The standard heat of combustion is shown in the following chemical equation
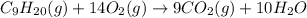
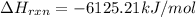 . If 130 g of nonane combusts , how much heat is released?
. If 130 g of nonane combusts , how much heat is released?
Answer: 6211.21 kJ
Step-by-step explanation:
Heat of combustion is the amount of heat released on complete combustion of 1 mole of substance.
Given :
Amount of heat released on combustion of 1 mole of nonane = 6125.21 kJ
According to avogadro's law, 1 mole of every substance occupies 22.4 L at NTP, weighs equal to the molecular mass and contains avogadro's number
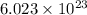 of particles.
of particles.
1 mole of nonane
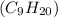 weighs = 128.2 g
weighs = 128.2 g
Thus we can say:
128.2 g of nonane on combustion releases = 6125.21 kJ
Thus 130 g of
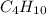 on combustion releases =
on combustion releases =
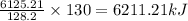
Thus the heat of combustion of 130 g of nonane is 6211.21 kJ