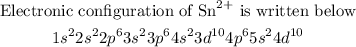Explanation
What to find? The electronic configuration of tin ion
Tin is an element with an atomic number of 50.
NB: Atomic number = number of proton
Hence, the number of proton of tin = 50
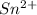
The above tin ion means it has lost two electrons, which make it has a total electron of 48
To write the electronic configuration of tin ion, we will be using the SPDF Notation
spdf are names given to orbitals that hold electrons in atoms.
s - orbital
p- orbital
d- orbital
f -orbital