Answer:
We must add 400 ml of water.
Explanation:
We can use the following equation:
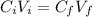
Where:
- C(i) is the initial concentration of the solution (80%)
- C(f) is the final concentration of the solution (30%)
- V(i) is the initial volume (150 ml)
- V(f) is the final volume
Now, we just need to solve the equation for V(f).
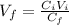
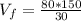
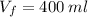
Therefore, we must add 400 ml of water.
I hope it helps you!