1) Concentration of Na+
1.1- List the known quantities.
Molarity: 0.304 M Na2SO4.
Volume: 15.0 mL
M= mol/L
1.2- Set the equation
The ion-molecule ratio is 2 Na atoms: 1 molecule Na2SO4.
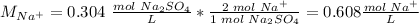
The concentration of Na+ is 0.608 M.
2) Concentration of SO4 (2-)
2.1- List the known quantities.
Molarity: 0.304 M Na2SO4.
Volume: 15.0 mL
M= mol/L
2.2- Set the equation
The ion-molecule ratio is 1 SO4 (2-): 1 molecule Na2SO4.
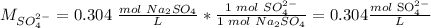
The concentration of SO4 (2-) is 0.304 M.
3) Concentration of K+
3.1- List the known quantities.
Molarity: 0.200 M KCl.
Volume: 34.6 mL
M= mol/L
3.2- Set the equation
The ion-molecule ratio is 1 K+: 1 molecule KCl.
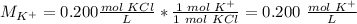
The concentration of K+ is 0.200 M.
4) Concentration of Cl-
4.1- List the known quantities.
Molarity: 0.200 M KCl.
Volume: 34.6 mL
M= mol/L
4.2- Set the equation
The ion-molecule ratio is 1 Cl-: 1 molecule KCl.
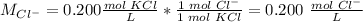
The concentration of Cl- is 0.200 M.
.