Answer: Theoretical yield of
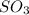 produced by 8.96 g of S is 33.6 g
produced by 8.96 g of S is 33.6 g
Step-by-step explanation:
To calculate the moles :
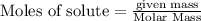
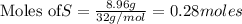
The balanced chemical equation is:
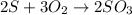
According to stoichiometry :
2 moles of
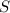 produce = 3 moles of
produce = 3 moles of
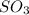
Thus 0.28 moles of
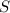 will produce=
will produce=
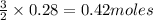 of
of
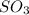
Mass of
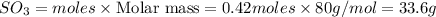
Thus theoretical yield of
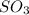 produced by 8.96 g of S is 33.6 g
produced by 8.96 g of S is 33.6 g