Answer:
26.67 mol HCl
Step-by-step explanation:
Al(OH)₃ + 3HCl → AlCl₃ + 3H₂O
In order to solve this problem, we need to convert Al(OH)₃ moles to HCl moles.
To do so we use the stoichiometric ratios of the balanced reaction:
- 8.89 mol Al(OH)₃ *
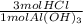 = 26.67 mol HCl
= 26.67 mol HCl
Thus 26.67 moles of HCl would react completely with 8.89 moles of Al(OH)₃.