Answer:
640.32 g
Step-by-step explanation:
2KClO₃ → 2KCl + 3O₂
First we convert KClO₃ moles to O₂ moles, using the stoichiometric coefficients:
- 13.34 mol KClO₃ *
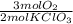 = 20.01 mol O₂
= 20.01 mol O₂
Then we convert O₂ moles to grams, using its molar mass:
- 20.01 mol O₂ * 32 g/mol = 640.32 g
So 640.32 g of O₂ are formed from 13.34 moles of KClO₃.