Answer: D. 1600 L
Step-by-step explanation:
According to avogadro's law, 1 mole of every substance occupies 22.4 L at STP and contains avogadro's number
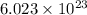 of particles.
of particles.
To calculate the moles, we use the equation:
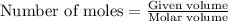
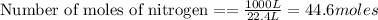
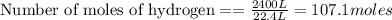
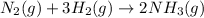
According to stoichiometry :
3 moles of
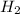 require 1 mole of
require 1 mole of
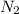
Thus 107.1 moles of
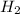 will require=
will require=
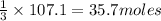 of
of
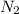
Thus
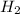 is the limiting reagent as it limits the formation of product and
is the limiting reagent as it limits the formation of product and
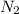 is the excess reagent.
is the excess reagent.
As 3 moles of
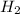 give = 2 moles of
give = 2 moles of
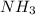
Thus 107.1 moles of
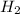 give =
give =
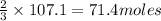 of
of
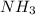
Volume of
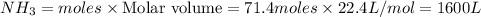
Thus volume of ammonia produced is 1600 L