During a chemical reaction the total mass remains the same. This means that the mass of the consumed reactants equals the mass of the created products. The number of atoms also remains the same but the molecules change.
Many meals have in their composition starch, a molecule that is formed by glucose monomers. When the human body uses glucose it transformes this carbon based molecule into water, carbon dioxide and energy.
The chemical reaction of this process is the following:
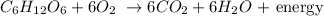
As we can see the number of each atom that conforms the reactants equal those of the products:
in total (both in the reactats and the products):
6 carbon atoms
12 hidrogen atoms
18 oxigen atoms
In this reaction glucose and carbon dioxide are both carbon based molecules