Answer:
7.5 g of hydrogen gas reacts with 50.0 g oxygen gas to form 57.5 g of water.
Step-by-step explanation:
Here we have the check if the mass of the reactants is equal to the mass of the products.
Reactants
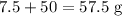
Products
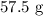
The data is consistent with the law of conservation of matter.
Reactants
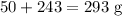
Products
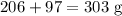
The data is not consistent with the law of conservation of matter.
Reactant
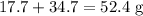
Products
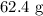
The data is not consistent with the law of conservation of matter.
Only the first data is consistent with the law of conservation of matter.