Reactivity series:-
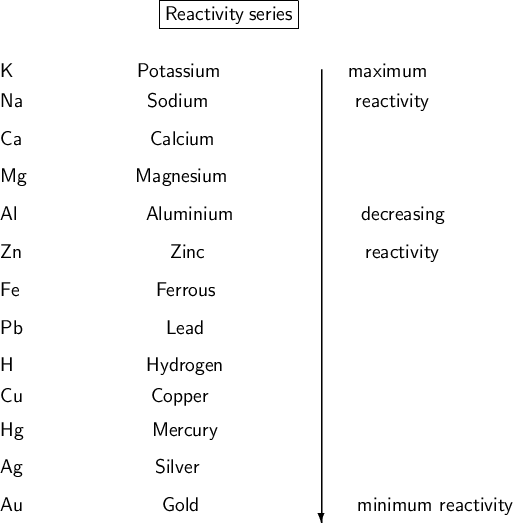
#1
Ag is found as too less reactive so
#2
No element in universe can displace potassium (K) from its solution as it's most reactive .
#3
Fe is also a reactive metal.
- Only Cupper is less reactive than it
So
#4
- Only Calcium can displace Mg
#5
- Mg and Ni can do ,Aurum(Gold) is least reactive so can't
#6
#7
#8
#9
#10
#11
As Ca is third most reactive metal
#12