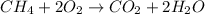
Based on the chemical equation 2 moles of oygen gas produces 1 mole of carbon dioxide, using this relationship we can set up an equation that would determine the amount of moles needed to produce 5.67 moles of carbon dioxide:
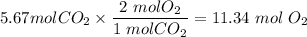
Answer: It will require 11.34 mol O2 gas.