Answer:
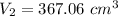
Step-by-step explanation:
Charles law states that volume of the gas is directly proportional to the temperature i.e.
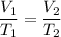
We ave,
V₁ = 300 cm³
T₁ = 40°C = 40 + 273.15 = 313.15 K
T₂ = 110°C = 110 + 273.15 = 383.15 K
Let V₂ be the new volume.
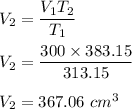
So, the new volume of the balloon is
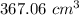 .
.