1) Find the formula mass of Al2O3.
Aluminum mass: 26.982 u.
Oxygen mass: 15.999 u.
Al2O3 mass = 2 * (26.982 u) + 3 * (15.999 u)
Al2O3 mass = 101.961 u.
2) Aluminum percentage.
Aluminum mass: 26.982 u.
Al2O3 mass = 101.961 u.
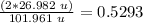
Moving the decimal point.
52.93%
The percentage of aluminum is 52.93%
3) Oxygen percentage.
Oxygen mass: 15.999 u.
Al2O3 mass = 101.961 u.
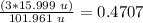
Moving the decimal point.
47.07%
The percentage of oxygen is 47.07%.
.