We are asked to find some characteristics of phosphorus. We start with the yu period group to which they belong in the periodic table. This information is found in the periodic table of elements.
Looking in the periodic table we find that phosphorus P is located in group VA (15) and in period 3.
The electronic configuration depends on the number of electrons of the element. Phosphorous has 15 electrons. We have an ion P^3-, this ion has three more electrons, so for this element, we will have 18 electrons. The electronic configuration will be:
The short notation depends of another element, in this case of Ne, so the shot notation will be:
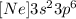
The valence level is the last energy level, we see that the greatest coefficient is 3, so the valence level is 3
In summary, we have that the answer will be:
Orbital Diagram: Drawn in the picture
Long Notation: 1s2 2s2 2p6 3s2 3p6
Short Notation: [Ne] 3s2 3p6
Valence Level: 3
Group on Periodic Table: 15 or VA
Period on Periodic Table: 3