Answer:
C. 3.33 moles
Explanation:
Ammonia decomposes
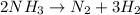
We have to find the moles of hydrogen formed in 2.22 moles of ammonia.
From the reaction
2 moles of ammonia gives 3 moles of hydrogen
1 mole of ammonia produce hydrogen=3/2 moles
2.22 moles of ammonia produces hydrogen=
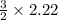 moles
moles
Number of moles of hydrogen formed by decomposition of 2.22 moles of ammonia
=
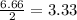 moles
moles
Hence, 3.33 moles of hydrogen are formed in 2.22 moles of ammonia.
Option C is true.
C. 3.33 moles